Describe in Your Own Words How to Prepare 100.0 Ml
Prepare five standard solutions in the 1x106-9x10-6 M range into five 1000 mL volumetric flasks. Complete to the mark with distilled water.

Solved Describe In Your Own Words How To Prepare 100 0 Mathrm Ml Of A 0 85 Mathrm M Solution Of Sodium Chloride
23Na 36Cl 59NaCl 85 mol NaCl x 59 gmol 50 gL or 50g NaCl for 110 L 100ml Weigh 50 g of salt dissolve in water.

. At a given temperature what factor determines the rates at which different molecules undergo diffusion. Describe in your own words the process of diffusion. So the solution is prepared by the addition of 481 ml total Mueller solutions.
What volume of water would you add to 1500 mL of a 677 M solution of nitric acid in order to. What is the molarity of a solution that is made by diluting 5000 mL of a 474 M solution of HCl. Weigh out 5844 g NaCl.
Place the NaCl in a 1-liter volumetric flask. Preparation of Calibration Standards. In your own words explain why osmosis occurs when distilled water and seawater are separated by a semipermeable membrane.
Kelly opened a savings account 14 years ago. Then measure the pH of the solution using a pH probe. The calculator uses the formula M 1 V 1 M 2 V 2 where 1 represents the concentrated conditions ie stock solution molarity and volume and 2 represents the diluted conditions ie desired volume and.
A 0100 00355 molL 00645 molL. In the given procedure 1000 mL of 0125 M KCl solution is prepared. Write the complete formula and solution in a padpaper.
Dissolve 111 g of CaCl 2 in sufficient water to make 100 ml of solution. What would be the concentration of a solution made by diluting 450 mL of 42 M KOH to 250 mL. Avoid storage of calibration solutions.
Use the standard Methylene Blue to accurately prepare a 0001M stock solution in a 1000 mL volumetric flask. You are given solid Na2CO3 distilled water and a 500 mL volumetric flask Describe how to prepare the required solution showing all calculations and describing each step. For example to make 100 ml of 01 M CaCl 2 solution use the previous formula to find out how much CaCl 2 you need.
ML of 0333 M solution. Pg 209 3 letter b. First week only 499.
In your own words describe how you would prepare 500 mL of 02 M NaOH from 6. Grams of CaCl 2 01 x 11091 x 100 1000 111 g. Solution for Describe how to prepare 10000 ml of a 0250 M sodium chloride NaCl solution using sodium chloride powder.
REACTIONS - Chemical reactions proceed on an ATOMIC basis NOT a mass basis. The account earned 3 interest. Describe in your own words how to prepare 1000 ml of a 085 m solution of sodium chloride.
Gas molecules originally in a confined area will spread evenly into the Slavs available to them. Add a small volume of distilled deionized water to dissolve the salt. An experiment in your laboratory requires 500 mL of a 0200 M solution of Na2CO3.
V1 800 mL T1 27C T2 77C V2. Describe the behavior of the following pair of substances as soluable insoluable miscible or immiscible. Decide on and prepare dilution solution eg.
Use Charless law to solve. Swirl the flask and then top. Describe in your own words how to prepare 1000 mL of a 085 M solution of sodium chloride.
For example adding 50 mL of ethanol to 50 mL of water will result in a total volume that is less than 100 mL. Problems that ask you to prepare a solution that has a certain molarity and volume starting from a stock solution can always be approached using the equation for dilution calculations. 98 CHEMICAL CALCULATIONS CONTINUED.
Describe in your own words how to prepare 1000 mathrmmL of a 085 ma 0032 What volume of 025 M HCl solution must be diluted to prepare 100 L of 004. She has not made any deposits or withdrawals since. The solution dilution calculator tool calculates the volume of stock concentrate to add to achieve a specified volume and concentration.
1000 mL of 25 M HCL from 118 M HCL. In the first method prepare a solution with an acid and its conjugate base by dissolving the acid form of the buffer in about 60 of the volume of water required to obtain the final solution volume. Take 999 mL of 0500 M sodium sulfate and add enough water to make the total volume equal 150.
Next slowly add your 4 mL of stock solution sulfuric acid. To prepare the 10 mL of 2 M solution you must first transfer about 5 mL of distilled water into your 10 mL volumetric flask. Calculate the molarity of the following solution.
Start your trial now. It is actually closer to 96 mL. Fill the flask to the 1 L line.
There are a couple of ways to prepare a buffer solution of a specific pH. Prepare 1 liter of 100 M NaCl solution. 00355 mol of acetic acid and 00645 mol of sodium acetate is required to prepare 1 L of the buffer solution.
That is going to 40 481 L. If her initial balan. First calculate the molar mass of NaCl which is the mass of a mole of Na plus the mass of a mole of Cl or 2299 3545 5844 gmol.
If using centrifuge tubes can use calibration solution direct from tube. Pour into 100 ml. Matrix solution 1 acid etc and use instead of ultra clean water as appropriate.
Label it as Methylene Blue Stock. Now you can make your solution. The amount of water needed will be slightly less than 100 ml.
When youre diluting a solution youre essentially increasing the volume of the solution by keeping the number of moles of solute constant and by. How many mL of the original solution will we need to dilute. So to prepare The given solution 4821 ml of volume that is added to Mueller solution.
According to chegg guidelines we will answer only the first question 1. In your own words describe the steps you took to make your solution. Prepare 1500mL of 05N H2SO4 solution with specific gravity of 184gmL and assay of 97.
In order to understand how buffer solutions maintain a constant pH let us consider the example of a buffer solution containing sodium acetate and acetic acid. Heres my take on this problem. So that means we can say there to prepare The given solution 41 this is enamel.
A 2500102M solution of NaCl in water is at 200C. Therefore when preparing volumevolume percent solutions it is always better to dissolve the solute in solvent and then add additional solvent to bring the total solution volume to the desired final value. Can pre-rinse beaker with stock solution to clean.
From the calculation you need to pipette 4 mL of the 5 M sulfuric acid solution to prepare 10 mL of 2 M sulfuric acid solution.

Solved A Describe How You Would Prepare 100 0 Ml Of 0 200 M Chegg Com

Ppt Ch 13 Solutions Sec 2 Pg 467 1 11 Powerpoint Presentation Free Download Id 441003
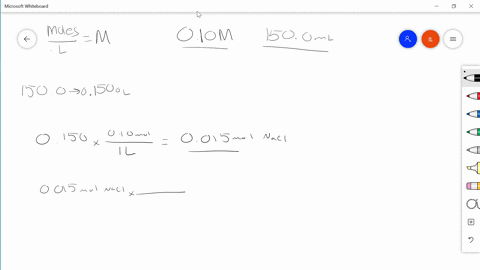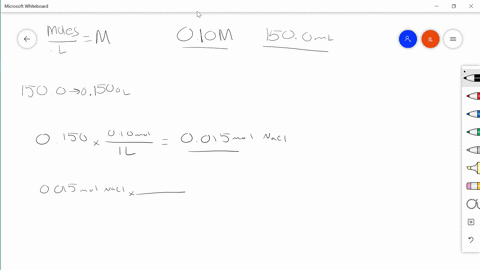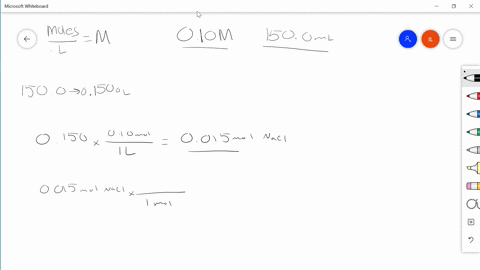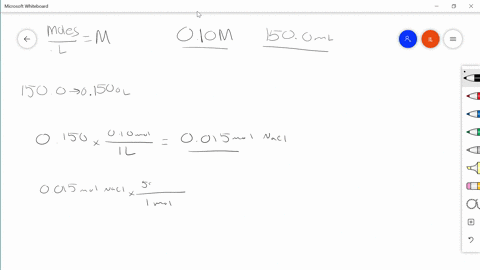
Solved Describe In Your Own Words How To Prepare 100 0 Mathrm Ml Of A 0 85 Mathrm M Solution Of Sodium Chloride
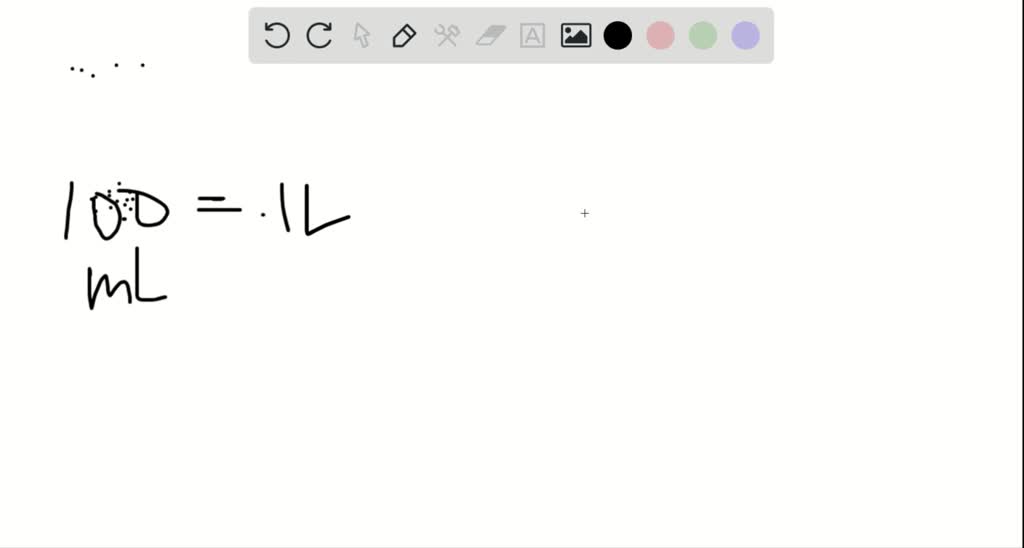
Solved Describe In Your Own Words How To Prepare 100 0 Mathrm Ml Of A 0 85 Mathrm M Solution Of Sodium Chloride
No comments for "Describe in Your Own Words How to Prepare 100.0 Ml"
Post a Comment